A recurring series focusing on plant cultivation by university researchers
A nutrient monitoring program can help ensure you provide plants what they require for optimal fertility. Several nutrient monitoring techniques allow you to analyze the pH and electrical conductivity (EC) status of the plant and to troubleshoot potential issues before they occur. In the August 2019 issue of Cannabis Business Times, we highlighted one of these techniques—the PourThru method, which North Carolina State University researchers adapted for greenhouse crops.
Alternative techniques some growers and commercial labs use are the 1:2 monitoring and the saturated media extraction (SME) methods, which test soilless substrate from plants grown in containers in greenhouses, indoors or outdoors. These two nutrient monitoring techniques involve removing a small sample of the substrate from multiple pots, diluting it with water, and then analyzing for pH and electrical conductivity (EC). These two metrics reveal the total quantity of dissolved fertilizer ions in the pot solution (EC) and how those ions will be available to the plant (pH). The primary difference between the 1:2 and SME procedures is the amount of water added to wet the substrate. Deciding which troubleshooting technique—PourThru, 1:2 or SME—is best for your operation comes down to preference and past training. Though limited, SME and 1:2 are destructive to roots, while PourThru is not.
Before expanding upon the procedures for 1:2 and SME, it is important to examine different irrigation strategies and how this will impact where you take your sample from the pot.
Step-By-Step Sampling Procedures
The 1:2 and SME nutrient monitoring systems consist of multiple straight-forward steps.
1. Pick three to five representative plants. It is important that your monitoring program includes three to five plants per section in your greenhouse or indoor operation (Fig. 1). A section is defined as a distinct region, climate, stage of plant development, or growing condition. Sections can also be comprised of individual cultivars. For example, if you have a cultivar that produces a lot of biomass and another cultivar that is more compact, you would want to sample each cultivar separately to ensure that each crop is being provided the nutrients it needs. The smaller cultivar could have a higher EC due to a lower use of fertilizer ions, while the larger cultivar could experience nutrient shortages due to greater biomass production.

2. Collect a substrate sample. As we noted in the July 2020 issue of CBT, fertilizer salts accumulate in the pot based on the irrigation method used (Fig. 2). If top irrigation is delivered, then the fertilizer salts tend to accumulate at the bottom one-third of the pot. If bottom or flood irrigation is provided, salts will accumulate at the top one-third of the pot. To account for the accumulation difference, two different approaches are possible.
a. Total root zone profile. A representative sample is taken by removing a slice of the substrate from the entire top to bottom profile of the pot (Fig. 3a). This allows the mixing of the top, middle, and bottom portions of the substrate profile and reflects the total environment that the roots are experiencing.

b. Middle root zone profile. This modified technique only takes a substrate sample from the middle region of the pot (Fig. 3b). By employing this technique, this sample is considered to be an average environment the roots encounter.
With either sampling method, you need to combine substrate samples from three to five pots to have at least 1 to 2 cups (250 mL to 500 mL) of sampled substrate (Fig. 4).

Remove debris such as plant roots and large substrate components (Fig. 5).
3. Dilute the substrate with water. Next, the substrate needs to be saturated and then rest for 30 to 60 minutes, whether you choose the 1:2 or SME method. The primary difference between the 1:2 and SME procedures is the amount of water added to dampen the substrate.

a. 1:2 Dilution. The 1:2 technique uses one part substrate and two parts distilled water to create a slurry, or a mixture of solids denser than water suspended in liquid (Fig. 6a). Combine those portions (Fig. 6b) and then allow the solution to sit for 30 to 60 minutes before testing.
b. SME Dilution. The SME technique is a bit more detail oriented. The goal is to create a wet paste solution, so the technique requires that just enough water is added (Fig. 7a) to achieve a paste (Fig. 7b). (If too much water is added, the substrate pH reading will be unaffected, but excess water will dilute the EC values obtained and will result in an incorrect lower EC level). The amount of water added is typically less than 50% of the volume of the substrate. Allow the solution to sit for 30 to 60 minutes before testing.

4. Calibrate your meter. Next, calibrate your pH and EC meter. The meter readings are only as accurate as your last calibration. Many meters are available on the market; however, any meter you purchase should have both a pH and EC function. Consult your manufacturer or your manual for instructions on how to calibrate the instrument (Fig. 8).
5. Refill the void in the pot. While waiting for the 1:2 or SME dilution sample to be ready, replace with new substrate the substrate that was removed sampling (Fig. 9).

6. Analyze your sample. Now that you have the substrate in a container, it is time to analyze it (Fig. 10). Follow the instructions on your meter to obtain both your pH and EC measurement. For the most accurate results, ensure the numbers on your meter reach a stable reading.
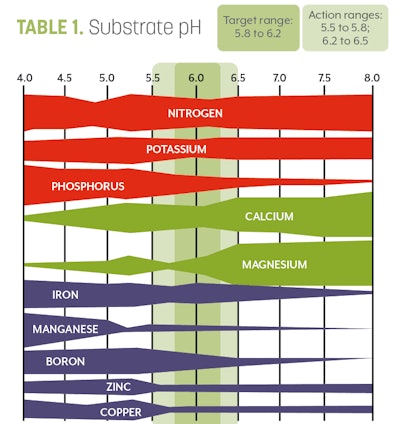
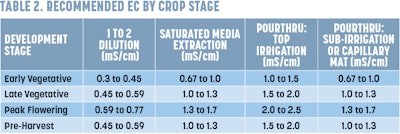
7. Interpret your results. You should have two numbers from your unit. The first will be a pH reading. The optimal pH and EC for cannabis can be found in Tables 1 and 2, respectively.
Corrective Procedures
Once you take the readings, determine if the values are outside of the acceptable ranges. If the pH or EC values have drifted out of those ranges, then take corrective measures to ensure that the fertility program is back on track and prevent plant damage.
For strategies to correct low and high pH, see the article, “New Research Results: Optimal pH for Cannabis” in CBT’s March 2019 issue.
To learn how solution and substrate EC can be used to enhance cannabis growth, see “Optimizing Electrical Conductivity (EC)” in CBT’s April 2019 issue.
As always, remember to recheck your substrate pH and EC within a few days to determine if reapplications are needed.
Summary
By monitoring your pH and EC through a nutrient monitoring program such as the 1:2 or SME methods outlined here or with the PourThru method covered in an earlier CBT article, you will be able to determine nutrient disorders before they become an issue. This will not only save you time but will also allow you to provide nutrients in a more precise and economical fashion.