Justin Gover, the CEO of GW Pharmaceuticals, told CNBC his company's new drug will pave the way for other medications derived from marijuana.
On Monday, the Food and Drug Administration approved the company's new epilepsy drug, Epidiolex, the first cannabis-based drug the U.S. regulator has approved.
"This is an approval, not just a landmark for Epidiolex and for patients who may benefit from this specific medication, but also a strong belief from this company that cannabinoids have a bright future in the form of other drug candidates derived from the cannabis plant across a range of other disease areas," Gover said Tuesday on "
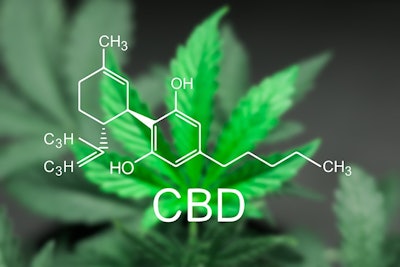
The medication is made from cannabidiol (CBD), one of hundreds of molecules found in marijuana plants. However, it is not known to produce a high. In fact, the drug concoction contains less than 0.1 percent of tetrahydrocannabinol (THC), the substance that induces a high.
Top photo courtesy of Adobe Stock
























