With the exploding legalization of cannabis for medical and recreational use in the U.S., consumers are demanding a growing range of new and different products for legal consumption. Many consumers prefer the experience of cannabis vaping products, which has contributed to a booming industry. As with any type of inhalation use, whether it be traditional smoking or vaping, consumers, manufacturers and retailers have a natural interest in safety.
Safety concerns about vaping products have proliferated after the lung injury (EVALI) outbreak in 2019, and a wave of lawsuits in the nicotine vaping industry related, in part, to the use of artificial flavorings. Some in the cannabis industry might assume that safety concerns about artificial flavorings are unique to the nicotine vaping industry, but recent studies and government regulations have specifically identified the use of artificial flavorings in cannabis vapes as a risk vector for the industry.
A Growing Problem
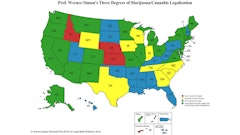
Legalization of cannabis itself is only one aspect of a multi-branched family of hemp and cannabis legislation that have been approved by states. Even among states that have not legalized cannabis, many have chosen to permit cannabidiol (CBD) for patients, separate from CBD derived from federally compliant hemp.
With the explosion of newly available cannabis products, accessory manufacturers have quickly responded with novel products for consumption, particularly for inhalation, which traditionally had been dominated by smoking. Similar to the explosive growth of e-cigarettes, the use of vaping devices to consume cannabis flower and oil has skyrocketed in recent years, with cartridge sales between 24% and 30% across state legal markets in 2020, according to data from LeafLink.
The popularity of vaping products in the cannabis industry is easy to understand. Vapes provide portability, ease of use, cleaner consumption without ash, and access to flavored cartridges. However, despite rising sales, cannabis consumers may be dismissing some of the red flags that have been identified with vaping in the tobacco e-cigarette industry, assuming that the differences between tobacco and cannabis products provide relative safety. Yet cannabis cartridges may have more in common with nicotine vaping products than consumers and retailers imagine, due to the lack of federal and consistent regulations and testing of artificial flavorings in cannabis vapes.
“What kept these toxins from flooding into the legal THC vape supply? Only the good conscious of many licensed vape cartridge manufacturers—and a bit of luck,” according to an article from Leafly investigating THC vape rules. Although flavorings, thickeners and other additives are much more problematic in the illicit market, there are still flavorings and terpenes from sources other than cannabis that make their way into the legal market.
Concerns about artificial flavors in tobacco vapes arose over time. Just as the number of cigarette users began to dwindle, vaping devices and e-cigarettes containing nicotine brought resurgent growth into the nicotine industry as an alternative to cigarettes, allegedly without the most harmful effects of smoking. And just as with cannabis vapes, nicotine vaping products also promised a fresh experience for new (and younger) users, through the proliferation of flavored vapes. But novel risks also arrived with the renewal in the tobacco industry that vaping products provided.
The Problem with Fake Flavors
While many of the artificial flavors used in nicotine vapes have long been recognized as safe ingredients in food products, a growing body of scientific literature suggests that inhalation of some of these same ingredients can harm lungs. Based on this research, the nicotine vaping industry is combating a raft of lawsuits, alleging, among other things, harmful exposure from inhalation of artificial flavors. More than 900 lawsuits have been filed against e-cigarette manufacturers, including JUUL Labs, Inc. (Juul). The consumers in these suits claim that Juul sought to develop and market a product that would create and sustain nicotine addiction without the stigma associated with cigarettes, through the use of appealing new flavors. Some of these flavors include vanilla, cool mint, cool cucumber and mango, which consumers claim contain artificial flavors and ingredients not safe for inhalation.
The consumers in the Juul lawsuits cited a 2016 study analyzing the ingredients in some of the most popular vaping flavors that found the concentration of artificial flavoring in nicotine vaping e-liquids to be of toxicological concern, particularly where e-liquids incorporated flavors using diacetyl, acetyl propionyl (AP) and benzaldehyde.
Diacetyl and AP are chemicals called diketones. Diketones are naturally present in numerous foods, including butter and beer, and artificial diketones are one of the key components in artificial butter flavorings. Before the advent of artificially flavored e-liquids, scientific literature suggested that occupational inhalation exposure to diacetyl in the food production industry was associated with respiratory disease, including exertional dyspnea and obliterative bronchiolitis (BO). BO is a rare pulmonary disease that is characterized by inflammation, narrowing, or obliteration of bronchioles in the lung. Because of its physical effects on the bronchioles, BO is often referred to as “popcorn lung.” Investigation of the connection between diacetyl and BO in food workers began in 1985, when the National Institute for Occupational Safety and Health (NIOSH) first visited a microwave popcorn factory to investigate two young, previously healthy, non-smoking employees had been diagnosed with BO after working in the mixing room of the factory. NIOSH noted the presence of diacetyl and other chemicals used in the factory but could not pinpoint the cause of the employees’ sudden illness.
In 2000, NIOSH investigators visited and inspected a Missouri microwave popcorn plant after Missouri health officials notified OSHA that nine workers from that plant had been diagnosed with BO. After a three-year study of those workers’ occupational exposures, NIOSH concluded that inhalation of butter flavoring chemicals poses a serious risk for occupational lung disease. By December 2003, NIOSH had issued a safety alert to businesses that may have used butter flavoring, suggesting safeguards like the use of personal protective equipment, and requesting the employers notify and warn workers of the risks.
As a result of government research investigating suspected links between diacetyl and other diketone flavorings to lung disease, the food industry now monitors and protects workers from inhalation risks when workers use artificial flavorings. Consistent with that concern and knowledge of the increasing use of artificial flavors in e-liquids, the Food and Drug Administration (FDA) and the Flavor Extract Manufacturers Association (FEMA) have issued guidance stating that the use of artificial flavors in vaping products of any kind should be rigorously studied before they are used in e-liquids. Despite this guidance and growing concerns about artificial flavors in the industry, it remains a normal practice for manufacturers and retailers to sell e-liquids and cartridges (whether nicotine or cannabis-based) without a full ingredient list. In fact, the research indicates that even where manufacturers have advertised the lack of diacetyl or AP in their e-liquids, these ingredients are still frequently detected in product samples.
As awareness has grown about the potential inhalation risks associated with artificial flavors, many e-liquid manufacturers and retailers have taken steps to distance themselves from diacetyl and AP. Some have also provided statements that their liquids do not contain these chemicals. However, a 2015 study investigated the diacetyl and AP content in a number of sweet-flavored nicotine vaping liquids and discovered that many liquid vaping cartridges are exposing users to these very ingredients. The study examined sweet flavored vaping liquids from European and U.S. manufacturers including butter, toffee, milk, cream, chocolate, and coffee flavors. In fact, diacetyl was detected in 110 of the 159 samples, and AP was detected in 53 samples. Fifty-two of the samples had levels of diacetyl that exceeded the limits for occupational exposure recommended by NIOSH. Notably, diacetyl and AP “were detected in samples coming from manufacturers who clearly stated that they were not present in their products,” according to study results published in Nicotine & Tobacco Research, indicating that the chemicals were either used deliberately, were components of e-liquid ingredients unknown to the manufacturers, or otherwise were merely contaminants or byproducts.
The Deal with Cannabis
Just like nicotine vaping products, cannabis vaping products offer a wide variety of flavors, including fruit, candy and herb. Many retailers and consumers would not expect these products to contain harmful flavorings. However, a casual online product search reveals that CBD and THC cartridge producers include among their ingredients “food-grade flavoring” and “strawberry flavoring” of unknown and non-specified sources. Cartridges are often marketed for their fruity or otherwise sweet flavors, although some may be naturally derived from the cannabis strain itself.
Experts recently raised concerns about off-label components in grape-flavored cannabis vapes in Oregon, noting in comments to the Oregon Liquor Control Commission that by vaping artificially flavored cannabis products, consumers were being exposed to “a plethora of dangerous compounds.” These concerns led Oregon to issue a new rule in late 2020 prohibiting the off-label addition to vapes of non-cannabis derived substances, such as artificial flavorings or other chemicals that affect a cannabis vaping product’s “consistency, texture, or viscosity.” Not only does Oregon’s new rule require identification of artificial flavor additives in cannabis vaping products on labels, the rule also requires the certification by the manufacturer that such additives are intended for human inhalation.
The rule, which takes effect April 1, 2021, for manufacturers and July 1 for processors and licensees, defines a “non-cannabis additive” as a “substance or group of substances that are derived from a source other than marijuana or industrial hemp,” which also includes terpenes such as myrcene, limonene or linalool that are not extracted from cannabis.
One lesson the tobacco vaping industry learned was that it could not assume that diketones like diacetyl or AP were not present even in allegedly “diketone-free” e-liquid components. Similarly, the legal cannabis industry cannot assume that its products are 100% free of artificial flavorings or non-cannabis derived terpenes that could pose risks to consumers. As more consumers turn to vaping as their preferred method of cannabis use, and as diacetyl and AP litigation continues to develop, cannabis industry manufacturers and retailers should take additional measures to ensure their protection. Even cannabis growers should ensure their contracts with e-liquid component manufacturers include indemnity agreements to hold them harmless should litigation arise. Of course, cannabis itself is not the component of vaping e-liquids that raises safety concerns, and many manufacturers use only oil derived from cannabis in products. Nevertheless, this fact alone does not provide any reassurance that even a cannabis grower would not be pulled into future lawsuits if widespread litigation developed in the cannabis industry centered on vaping products. In fact, in the Juul lawsuits, not only Juul, but also numerous retailers and e-liquid manufacturers were named as co-defendants.
Cannabis manufacturers should also seriously consider contractually requiring component flavor or botanical terpene manufacturers with whom they do business to certify (ideally with certificates of analysis) that their products do not contain the artificial flavorings only previously used in the food industry. And as has become clear through the scientific literature, even these assurances may not guarantee the absence of these chemicals in a final product. As such, these manufacturers and retailers should also require vendors to identify any diketone components capable of producing diacetyl or AP as byproducts. Only through cautious and forward-thinking risk management will the future of the cannabis vaping industry be protected from the unforeseen negative impacts of artificial ingredients in the tobacco vaping industry.