
In the world of indoor cannabis cultivation, every drop of water matters.
Growers understand the importance of water quality and sustainability in achieving exceptional horticultural outcomes.
One often-overlooked source of high-quality water is condensate water recovery. In this guide, we'll explore how it can benefit indoor cultivation processes.
What is Condensate Water Recovery?
Condensate water is a valuable byproduct of heating, ventilation, air conditioning, and dehumidification (HVACD) systems. It is essentially distilled water that collects on the evaporator coils during the temperature and humidity control processes. This water then drips off the coils into a drain pan and is typically drained to waste. As water process engineers, we typically refer to and label these waste streams as Condensate Drain Water (CDW).
Water Quality Fundamentals for Indoor Growers
Before addressing how to capture and evaluate condensate water, it’s important to understand the fundamental water properties that affect Water Quality (WQ). Three of these properties include:
2. Electrolytes
Electrical Conductivity (EC) or its derivative measurement, Total Dissolved Salts (TDS), is a crucial parameter for hydroponic growers. In simple terms, this parameter assesses how well water can conduct electricity, or conversely, how resistant it is to electrical flow. EC is directly related to the quantity of electrolytes or salts dissolved in the solution and is commonly expressed in millisiemens per centimeter (mS/cm).
Microbial Load refers to the number of microorganisms present in a water supply and indicates the biological water quality. In the field of microbiology, this quantity is measured in Colony-Forming Units (CFUs). Furthermore, the total concentration of microorganisms is measured in CFU/ml, providing an estimate of viable bacteria or fungi present in a unit volume of a water sample, encompassing both pathogenic and non-pathogenic organisms.
Focusing on these three fundamental factors will help cultivators ensure that their water quality meets the requirements for healthy plant growth.
Water Quality of Condensate Recovery Water
Theoretically, the condensation process results in water that is relatively “pure” as measured by alkalinity or EC; however, the actual quality of condensate water can be influenced by several factors:
- Source
The source of the CDW matters. Condensate is typically formed on the fins of an evaporator, or “cold coil,” of an air-conditioning (AC) or dehumidification (D) system. It also arises as a by-product of natural gas combustion required for heating (H). These byproducts are typically devoid of alkalinity and require a neutralizing filter before being discharged into a drain system to prevent corrosion per International Plumbing Code (IPC) Section 803.
- Airborne Contaminants
One of the most significant challenges associated with condensate recovery is the potential for contamination by airborne agents. A ventilation (V) system regularly exchanges or circulates the air within an indoor plant environment (IPE), causing it to contact the heat exchangers, or "coils," in the air handling unit (AHU). Contaminants presented in the air may include dust, dirt, mold spores, pollen, Volatile Organic Compounds (VOCs) – including terpenes, and aerosols from foliar applications such as fertilizers, insecticides, and fungicides. These primarily organic contaminants accumulate on the coils and find their way into the collected condensate water.
- Corrosion Contaminants
Condensate water possesses a slight corrosive property toward metals, gradually dissolving the surfaces it encounters. Heat exchangers and drain pans can be constructed from various materials like stainless steel, galvanized steel, or aluminum, each with varying degrees of corrosion resistance and potential to release metallic ions like copper, iron, aluminum, lead, and zinc during this corrosion process.
- Drain Pan Contaminants
Over time, condensate drain pans in HVACD systems tend to foster microbial growth. This growth can lead to issues such as mold formation, bacterial growth, clogged drains, and associated overflow problems. As a result, it is recommended to regularly inspect, clean, treat, and sometimes replace these drain pans, as they can become vectors for contaminating the condensate source water. Cleaning agents, algaecides, biocides, and drain pan coatings are all potential sources of contamination in this context.
Moreover, it is essential to consider what else might accumulate in a drain pan and be discharged from an HVACD system. For instance, what if a refrigeration compressor experiences an oil leak, catches fire, or explodes? What if the HVACD system employs a water-glycol fluid mixture in its heat exchanger and experiences a leak? These scenarios highlight additional factors that can affect the quality of condensate water recovered by indoor growers.
Effect of Condensate Recovery on Primary Source Water Quality
With the foundation of how CDW water quality can vary, we are positioned to understand how it can affect the WQ of your water reserves. Let's cover the WQ effects on the three fundamental water quality parameters outlined above—namely alkalinity, EC, and microbial load.
Alkalinity Reduction
When the alkalinity of a primary water source is excessively high, growers will take steps to reduce it, either through neutralization, demineralization, or dilution processes. Dilution, or blending, to reduce alkalinity requires another source that is lower in alkalinity than the primary source, which includes CDW and rainwater sources. Because mixing can be a challenge, below is a simplified mixing example along with step by step calculations.
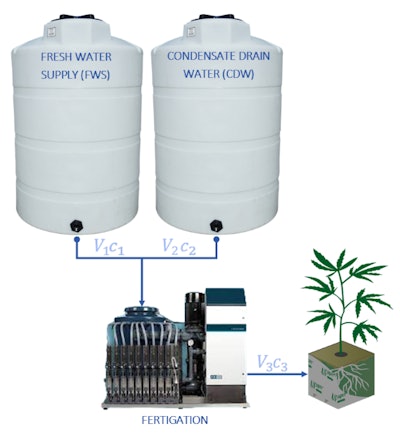

Figure 1: A simplified mixing problem with two collection tanks, packaged fertigation equipment, state variables, process flow diagram (PFD).
Furthermore, the amount of CDW collected from a cultivation operation will depend on several factors that are beyond the scope of this article; however, to simplify this mixing example we will assume that the IWS is made up of an equal proportion of FWS and CDW, establishing the following relationship:
V1 = V2 (Equation 2)
Substituting Equation 2 into Equation 1 simplifies the relationship between the volumes being mixed as follows:
V3 = 2V1 (Equation 3)
A solution's concentration (c) is how much mass (m) of a dissolvable substance (solute) is mixed with the volume (V) of another substance (solvent). In this alkalinity reduction example, the concentration of equivalent CaCO3 dissolved in water is characterized by the following equation:
c = m/V (Equation 4)
V = m/c (Equation 4.1)
Based on WQ tests of the FWS, we are empirically able to know that the alkalinity concentration of each source is as follows:
IWS concentration: c3 = TBD mg/L (eq. CaCO3 )
Now that we have established a proportional relationship between the mixing volumes and the upstream concentrations, we need a governing equation for mixing of two solutions of different concentrations to reduce the alkalinity of IWS. Therefore, by substituting equation 4.1 into each term in equation 1, we can derive the following equation:
V3 c3 = V1 c1 + V2 c2 (Equation 5)
Solving in terms of the IWS concentration (c3) we can rewrite the above equation as:
c3 = (V1 c1 + V2 c2)/V3 (Equation 6)
Substituting Equation 2 into Equation 6 simplifies the expression as follows:
c3 = (V1 (c1 + c2))/V3 (Equation 7)
Substituting Equation 3 into Equation 7 simplifies the expression as follows:
c3 = 1/2 (c1+c2) (Equation 8)
Therefore, the alkalinity of the mixed solution will be as follows:
c3 = 1/2 (104+0) mg/L = 52 mg/L eq. CaCO3
This simplified sample problem provides the step-by-step derivation of the dilution relationship, and respective calculations show that if the recovery rate is 50% of the supplied irrigation water, then neutralization and demineralization may not be required. Each site is going to have different source water quality (concentrations) and recover varying proportions of condensate water. This broken out math is meant to be a tool to easily calculate the effects of recovery based on unique sites, systems, and situations.
Electrolyte Reduction
When the conductivity of a primary water source is excessively high, growers will take steps to lower it, either through dilution or demineralization/desalination processes. Mixing sources to reduce conductivity requires an added source with a lower EC than the primary source. To show this effect, a similar simplified mixing problem is calculated below.
Based on water quality testing, we know that the EC of each source is as follows:
CDW concentration: c2 = 0.015 mS/cm EC
Therefore, using Equation 7 above we can calculate the EC of the mixed solution as follows:
IWS concentration: c3 = 1/2 (0.355 +0.015) mS/cm = 0.185 mS/cm EC
Microbial Contamination
Recovered condensate water presents a significant biosecurity risk to your cultivation operation. This is because organic contaminants accumulate in CDW based on the substances it encounters during the drainage and collection process that increase the Biochemical Oxygen Demand (BOD) / Chemical Oxygen Demand (COD), or how much oxidation is required to break down this organic material.
This continuous presence of organics, along with a constant trickle of oxygenated water, establishes the ideal setting for microbial growth and activity, essentially creating an aerobic reactor. This phenomenon becomes particularly pronounced when the collected water is stored for extended periods, allowing for aerobic digestion to take place. Therefore, when the concentration of microorganisms in a water source is excessively high, growers should take steps to sanitize, either through chemical treatment, Ultraviolet (UV) sterilization, or even pasteurization.
Editor's note: This is the first article in a series about condensate water recovery. Stay tuned to Cannabis Business Times for more on this topic.
Brad Hull, PE is a multi-disciplinary engineer and amateur horticulturist with 12+ years of experience planning, designing, building, and operating Controlled Environment Agriculture (CEA) enterprises in the United States.
As the Principal Engineer of Integrated Water Process, he works alongside cannabis growers to design, build, and maintain industry leading water management systems.
He holds a Bachelor of Science degree in Mechanical Engineering from Wright State University with a focus in renewable energy, as well as a Master’s in Business Administration degree from the University of Cincinnati with a focus in new venture creation. Brad is a registered Professional Engineer (PE) in the state of Ohio.

























