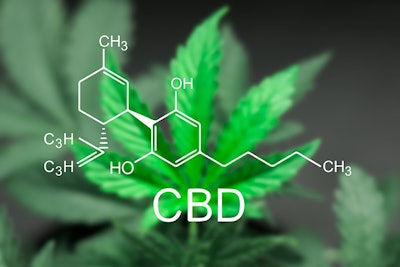
The Food and Drug Administration on June 25 approved for the first time a drug made from cannabidiol (CBD), a molecule derived from the cannabis plant. The drug, Epidiolex, was approved for the treatment of two types of epilepsy, Dravet syndrome and Lennox-Gastaut syndrome, that have been resistant to treatment.
Well-designed clinical trials have shown that the Epidiolex product of CBD can be helpful in reducing or eliminating seizures in these epilepsy syndromes.
While medical marijuana supporters may cite the FDA approval of Epidiolex as evidence of the benefits of marijuana, it is not an endorsement of any CBD or cannabis product. This product differs from most other CBD products available in cannabis dispensaries in that it is a highly concentrated and purified pharmaceutical grade medicine.

























