
President Biden’s May 16, 2024, announcement that the executive branch’s Office of Management and Budget (OMB) reviewed the DEA’s acceptance of the recommendation of the Department of Health and Human Services to change the federal classification of marijuana—has set the industry abuzz.
When cannabis becomes a Schedule III controlled substance, it will vacate the dreaded Schedule I classification of the Controlled Substances Act of 1970, where it has been alongside other illegal drugs, including LSD, ecstasy, and heroin.
Schedule III will place cannabis in the same medical classification as ketamine, anabolic steroids, acetaminophen (Tylenol) with codeine and certain barbiturates. But know this: A Schedule III designation makes cannabis a medical drug considered more dangerous and addictive than Schedule IV medications, which include Valium, Ativan, and Ambien, to name a few.
Take a look at the five federal classification schedules in the chart below.
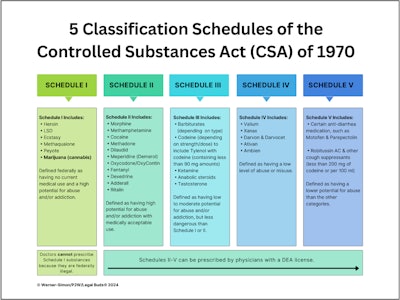
A Schedule III designation means that cannabis, federally criminalized since the 1970s and the administration of President Richard Nixon, will now become something physicians can prescribe to address medical ailments. (Currently, doctors in even state-legal regimes do not prescribe marijuana; they “recommend” marijuana for their patients—a distinction that keeps the DEA and federal law enforcement from accusing doctors of prescribing federally illegal substances.)
A Schedule III classification would be the second time in some 50 years that the federal government will have recognized that marijuana has medically therapeutic properties for use in patient populations. (The first time was in 2018 when the U.S. Food and Drug Administration (FDA) approved Epidiolex, an oral medication containing purified marijuana plant derivatives to treat severe forms of epilepsy and genetic seizure disorders.)
Rescheduling is a cataclysmic shift in federal drug policy. It changes the anti-cannabis trajectory that started in the Nixon era. President Nixon repeatedly ignored his hand-selected drug commission’s report, which recommended that marijuana be decriminalized. This view continued through the Trump administration, where President Trump’s attorneys general were vocally anti-cannabis, and the DEA repeatedly blocked federal scientific studies of the product. And in the rare instances during the last few decades when scientists were granted federal approval to conduct a scientific study into or with a Schedule I federally illegal drug, they were only authorized to conduct their studies with substandard cannabis provided to them by the federal government’s sole DEA-licensed cannabis grower at a university farm in Mississippi.
Biden’s Step-by-Step Changes to Longstanding Federal Government Anti-Cannabis Policy
The impending Schedule III designation will be the capstone in the Biden administration’s long-game, step-by-step reversal of federal drug policy. In 2021, shortly after Biden took office, the Biden administration’s DEA (without fanfare or press releases) started issuing multiple cannabis grower research agreements. Researchers circa 2021 were no longer required to use only the University of Mississippi’s federally authorized marijuana. The DEA began expanding the number of federally approved research registrants, permitting more researchers across the nation to both cultivate and study their own product.
As of October 2023, with the approval of Curia Wisconsin Inc., in Wisconsin, the federal executive branch (Biden’s) DEA has approved eight bulk cannabis suppliers for cannabis research, also operating in Arizona, Florida and Pennsylvania, as well as three in California, in addition to the University of Mississippi.
Other pro-cannabis medical research momentum came at the end of 2022, surprisingly from the federal legislators, when the U.S. Congress, with bipartisan support in the House and Senate, passed the Medical Marijuana and Cannabidiol Research Expansion Act. The research act’s dual goals are to expand cannabis studies of the plant’s potential health benefits and also to streamline the federal application process for scientific studies that have previously slowed the research process. President Biden signed the bill into law on Dec. 2, 2022.
The administration’s slow but steady embrace of cannabis is also evidenced by President Biden’s federal marijuana possession pardon proclamation in October 2022 and his December 2023 expansion, which made more cannabis offenders eligible for federal pardons.
When the Department of Health and Human Services (HHS), a federal cabinet agency, in August 2023 recommended to the DEA that it reclassify cannabis from an illegal drug to a Schedule III medication—it was a shocker but, it was not a done deal.
But now, in 2024, with the DEA accepting the HHS recommendation, and with the White House’s OMB on board, dramatic change is on the verge of happening. It is not the be-all-end-all; it is not full-blown legalization and decriminalization.
What rescheduling means is that the federal government is, to a degree, following the trends set by the states. As of press time, 24 states and two inhabited U.S. territories, plus the District of Columbia, have adopted recreationally legal regimes with coexisting medical programs. These places include California, Colorado, New Jersey, the American Commonwealth of the Northern Mariana Islands (CNMI), and most recently, as of the November 2023 elections, Ohio.
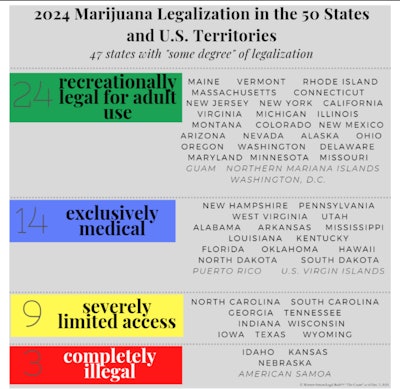
The above color block chart by Prof. Werner-Simon is based on data from the May 2, 2024, Congressional Research Service Report entitled “The Federal Status of Marijuana and the Policy Gap with the States.”
The Biden administration’s acceptance of cannabis as a medication correlates most directly with those states that begin the cannabis legalization process by becoming a medically legal state. Recall that California became the first state to legalize medical marijuana in 1996. And ultimately, 20 years later, in 2016, California legalized adult-use cannabis.
As the chart above reflects, 14 states, like Pennsylvania and Florida, remain exclusively medical. In fact, Pennsylvania was the first medically legal state to require medical research through designated Academic Clinical Research Centers, ACRCs, as part of its 2016 medical legalization legislation. But as Florida shows us (which medically legalized in 2016), transformation into a coexisting adult-use state no longer takes 20 years, as with California. The pace has quickened, and most medically legal states are more quickly “blossoming into” states with coexisting adult-use programs.
Florida has adult-use legalization on the upcoming November 2024 ballot. Voters there are polling just shy of the 60% needed to change the state’s constitution to enshrine the adult use of recreational cannabis. And Pennsylvania Gov. Josh Shapiro has called on Pennsylvania’s Legislature to legalize adult-use cannabis and even included anticipated adult-use tax revenue in his current state budget proposal.
This portends that the federal Schedule III medication designation is not the end of the federal legalization journey. It’s a toe tip into the legalization waters.
Forward Momentum Obscures the Elephant in the Room: Adult-Use Cannabis Would Still Be Federally Illegal
With all this forward momentum from the federal government and across the states, something significant is lost in the hubbub. The shift in federal drug policy by the classification of cannabis as a Schedule III medication does not federally legalize adult-use cannabis. The battle between the states (the incubators of cannabis innovation) and the federal government just now viewing the substance as something that doctors can prescribe—will persist. Much of the tension between the federal government and the stakeholders will be in the details, experts in the field say.
Dr. Lauren Simon, clinical professor of Family Medicine at the University of California, Riverside, and past president of the California Academy of Family Physicians, explains that rescheduling marijuana to Schedule III to be like ketamine and anabolic steroids still means that marijuana will be a federally controlled substance. “Schedule III drugs are typically prescribed by physicians but in very specific and limited medical circumstances,” she says. “I’m not sure how this changes things significantly other than the fact that physicians will not risk losing their DEA licenses to prescribe medicine.”
Judith Cassel, an industry veteran and marijuana access impact litigator at Hawke, McKeon & Sniscak LLP, and principal in the entity Cannabis Law PA, says, “The Drug Enforcement Administration’s (DEA) acceptance of Health and Human Service (HHS)’s request for reclassification of cannabis from Schedule I to a Schedule III medication predicts ‘a significant amount of work ahead before rescheduling is finalized. There will be a comment period and likely lawsuits will be filed.’” This is not the end of the story. Additionally, Dr. Simon wonders “if all the marijuana dispensaries will now hire or retain physicians to write prescriptions for patients to obtain medical marijuana products and what effect the ‘medicalization’ of marijuana will have on the nation's recreational dispensaries.”
Courtney Weber from the law firm of Foster Graham Milstein & Calisher LLP in Denver, and someone who studied marijuana law in law school, responds with this: “The bottom line is there's a lot of ambiguity, and until we have a better understanding of how the feds will regulate it and whether the adult-use market will get addressed in the process leaves a lot of fluidity in the process.”
What all experts agree on is that there remains a good deal of uncertainty with the transformation of an illegal drug into a federally approved medication. Plus, the timing of the changes could be impacted by the upcoming election.
Camilla Beldham, a teaching assistant in cannabis law at the University of Southern California’s Gould School of Law, says, “If all this downgrading (rescheduling) to a less severe federal schedule is not finished by the time President Biden is sworn in for a second term on January 20, 2025, or if former President Trump, whose two U.S. attorneys general were anti-marijuana and anti-cannabis research and cannabis company mergers, retakes office, all this federal legalization momentum can be squelched.”
Regardless, good things are on the horizon for other aspects of rescheduling. As attorney Cassel says, “The DEA’s acceptance of Health and Human Service (HHS)’s request for reclassification of cannabis from Schedule I to a Schedule III medication under the Controlled Substances Act (CSA) will provide many benefits to medical and cannabis providers.”
Let’s take a look at several of those benefits to those in the industry related to taxation, employment, bankruptcy, banking, federal trademarks and immigration:
(i) Taxation Benefits Expected for Cannabis Businesses as a Result of Rescheduling
Topping the list for the most significant change to the industry and profitability from rescheduling is federal tax code treatment. Economic studies have shown that cannabis companies in 2022 paid approximately $1.8 billion more in federal income tax than regular businesses because of Internal Revenue Code Section 280E, with some companies paying federal income tax at an effective tax rate greater than 70%. This results in unequal tax treatment for the industry.
As Cassel says, “For grower/processors and dispensaries, the dreaded 280E will be gone, freeing up funds for production.”
Professor Stacy Kline, a CPA and an assistant dean at Drexel University’s LeBow School of Business, who teaches the Emerging Business of Cannabis, echoes Cassel.
Kline, who has her students argue for and against 280E application in mock debates, says, “Currently, Internal Revenue Code Section 280E imposes a significant tax burden on cannabis companies by disallowing trade or business deductions for expenditures in connection with the illegal sale of drugs. Cannabis companies that cultivate, transport, or sell cannabis products cannot deduct any operating expenses other than inventory costs, i.e., cost of goods sold. Rescheduling marijuana as a Schedule III drug will result in significant federal income tax relief to cannabis companies and reduce their effective income tax rate to 21 percent, just like all other businesses.”
(ii) Employment Practices and Health Insurance Coverage Changes Anticipated as a Result of Rescheduling
Another expected change as a result of rescheduling will be in the area of employment and health insurance coverage. Cassel says, “When medical marijuana is rescheduled, patients will have this medicine covered by insurance plans, and taking it will not be an automatic termination of employment.”
(iii) Bankruptcy Benefits Anticipated for Cannabis Businesses as a Result of Rescheduling
Another federal privilege long denied to the cannabis industry is bankruptcy protection.
Weber, of Foster Graham Milstein & Calisher LLPC, explains that, with rare exceptions, state-legal cannabis companies and even ancillary (non-plant-touching) businesses that service the industry have been denied the privilege of starting over with a clean slate by the U.S. Bankruptcy Courts.
Weber says rescheduling is promising for the industry but that more guidance is needed on how cannabis will be regulated under Schedule III. “It is likely that rescheduling will help increase access to bankruptcy protections when an individual or business is engaged in state-legal medical marijuana plant-touching or ancillary enterprises,” she says.
According to Weber, bankruptcy courts should more readily find that medical marijuana businesses are no longer engaged in federally illegal activities, and so these businesses should be able to avail themselves of bankruptcy protection. “What happens to those involved in adult-recreational cannabis businesses as they would not be covered by the rescheduling of marijuana, will remain an open question,” Weber says. “That conduct could still be considered ‘federally illegal marijuana-related activity,’ which would likely mean bankruptcy denials.”
(iv) Access to Banks and Banking Services Will Result From Rescheduling
Heidi Urness, chair of the Cannabis Practice Group at the Seattle office of the Louisiana-based law firm of McGlinchey Stafford PLLC, who assists financial institutions with their cannabis programs, has informed views on rescheduling. Urness says that after rescheduling, a bank or credit union’s decision to serve the marijuana industry—at least for medical marijuana—will likely improve. However, given that recreational marijuana will remain federally illegal even after rescheduling, banks may not jump in to service all of the industry after rescheduling.
Urness says, “While, given the current tension between federal law and state-legal recreational and medical programs, there have been no reports of any financial institution having their charter revoked for banking cannabis in compliance with the 2014 FinCEN (Bank Secrecy Act Expectations Regarding Marijuana-Related Businesses) guidance, exposure to federal anti-money laundering and racketeering laws would still persist after rescheduling.”
Urness warns that risks, such as the loss of status as a federally insured depository institution as well as civil seizure and forfeiture of assets, mean that financial institutions still need more federal action—such as the passage of the SAFER Banking Act—to allow the cannabis industry to be “fully served” by financial institutions.
(v) Federal Trademark Possibilities Should Improve for Cannabis Businesses
Cannabis businesses cannot get federal trademark protection for their names and logos because of federal illegality. The U.S. Patent and Trademark Office (USPTO), in denying trademark protections to cannabis businesses, even looks at cannabis companies’ websites to support its denial of federal trademark protection. A typical USPTO denial uses language like this: “The evidence of record indicates that the items or activities to which the proposed mark will be applied are unlawful under the federal Controlled Substances Act.” (See below.)

But since rescheduling will make medical marijuana federally legal, this will, at a minimum, permit trademark protection for those in the medical aspect of the industry.
(vi) Immigration Benefits Will Manifest for Cannabis Businesses as a Result of Rescheduling
Cannabis employees in state-legal businesses and cannabis business owners who are not American citizens can be denied American citizenship due to their involvement in federally illegal conduct. While American citizens can take comfort in the safe harbor of state-legal regimes, the same protections do not apply to noncitizens in America. Werner-Simon, Federal lawyer magazine: https://issuu.com/federalbarassociation/docs/tfl_novdec2022-web pages 66-74.
Last year, the federal Ninth Circuit Court of Appeals ruled that a Washington state legally licensed cannabis business operator’s application for American citizenship was properly denied because the El Salvadoran-born Washington state resident was engaged in federally illegal activity.
The federal court upheld the U.S. Citizenship and Immigration Services (USCIS)’s denial of Maria Elena Reimer’s naturalization application because she operates a business that remains illegal under the Controlled Substances Act of 1970. “The CSA categorically precludes her from qualifying for naturalization” because, according to USCIS, engaging in federally illegal activity precludes a finding of “good moral character” required of all future citizens.
Veronica Jeffers, an immigration law professor at Southwestern Law School in Los Angeles, looks forward to changes in U.S. immigration law vis-à-vis marijuana.
Currently, the Immigration and Nationality Act renders a noncitizen who violates, conspires or attempts to violate “any law or regulation of a State, the United States, or a foreign country relating to a controlled substance, as defined by section 102 of the Controlled Substance Act” inadmissible. INA Sec. 212(a)(2)(A)(i)(II).
This includes certain marijuana offenses and clearly would prevent the permanent residence or citizenship of someone who owns or is employed by a state-legal marijuana business. Even though changes in immigration policy occur at glacier-like speed, a Schedule III classification would ultimately benefit noncitizens, many of whom seek to make the U.S. their home.
The Biggest Open Question: How Will Rescheduling Impact Cannabis Industries Legal in the States But Not Covered by Rescheduling?
As of today, here is the count of how many states and territories have some degree of legalization:
- Recreationally Legal (with coexisting medical legalization): 24 states and 2 territories (Guam and the Northern Mariana Islands) and Washington, D.C, a federal district;
- Exclusively Legal for medical use: 14 states and 2 territories (Puerto Rico and the U.S. Virgin Islands);
- Severely Limited Access: 9 states
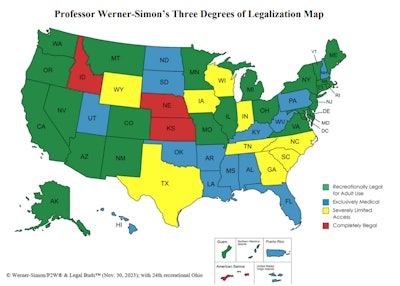
U.S. Map Explanation:
(i) adult-use states with coexisting medical regimes, like California (green on the map);
(ii) exclusively medical legalization for a plethora of medical conditions treated by any amount of THC product, like Pennsylvania (in blue on the map); and
(iii) those states that provide severely limited access (SLA) – including some states that call themselves “medically legal” but only permit a lone class or a few categories of people (such as patients who prove that they have intractable epilepsy) to use reduced THC, a limited amount of product and/or only CBD products (yellow on the map).
The degrees of legalization map (above) shows that over 98% of America’s 330 million inhabitants have some degree of access to cannabis. What impact federal rescheduling will have on the vast majority of Americans who already have access to state-legal product and/or who operate state-legal businesses, including cultivators, manufacturers and dispensaries, is an open question.
Conclusion
Rescheduling of cannabis is a significant step in changing 50-plus years of a peculiar and puzzling federal policy. The immediate benefits are pretty clear. Cannabis businesses, at least those who cultivate, dispense and manufacture medical cannabis, will fare better financially. Plus, medical patients will have access to a federally regulated product, which will bring with it federal standards and the ability to transport medical product across state lines.
But as with other aspects of life, change brings uncertainty. While the impending Schedule III is a hopeful start, cannabis’s new classification will result in more questions than answers. Industry stakeholders must remain vigilant and insist that policymakers address the outstanding concerns before the Schedule III designation is implemented.
_________________________________________________________________________
Julie A. Werner-Simon, a former federal prosecutor, is a legal analyst on the emerging business of cannabis at Drexel University’s LeBow School of Business and law professor adjunct at University of Southern California’s Gould School of Law and Drexel University’s Kline School of Law where she teaches “Marijuana Cannabis Law: History, Effective Business Practices and Degrees of Legalization.” She consults for the industry and can be contacted at www.persuade2win.com/contact.

























